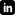Warm Congratulations to Jingbang for Winning the ISO 13485 "Medical Device Quality Management System" Certification Certificate
pcba15@fastpcba.cn
May 30, 2019 13:51:00 Warm Congratulations to Jingbang for Winning the ISO 13485 "Medical Device Quality Management System" Certification Certificate
Scope of Quality Management System Certification: Production of printed circuit board assemblies for electrocardiographs and endoscopes.
Passing the ISO 13485:2016 certification not only proves that under a quality-assured system, Jingbang Electronics can continuously provide high-quality products that meet the needs of medical device customers, but also indicates that our company has higher requirements for product quality standards. The successful passing of this ISO 13485:2016 certification is an recognition of our company's product quality and safety regulations!
Shenzhen Jingbang Electronics Co., Ltd. will continue to adhere to a refined management approach, further improve the management mechanism, and strive to create better and higher-end products! At the same time, it will enable the company to continue to develop in a better direction!
Passing the ISO 13485:2016 certification not only proves that under a quality-assured system, Jingbang Electronics can continuously provide high-quality products that meet the needs of medical device customers, but also indicates that our company has higher requirements for product quality standards. The successful passing of this ISO 13485:2016 certification is an recognition of our company's product quality and safety regulations!
Shenzhen Jingbang Electronics Co., Ltd. will continue to adhere to a refined management approach, further improve the management mechanism, and strive to create better and higher-end products! At the same time, it will enable the company to continue to develop in a better direction!
This is to certify that the Quality Management System of
Shenzhen Jingbang Electronics Co., Ltd. (Unified Social Credit Code: 914403006925114418), with the Operation Address at 3F, Building 1, No.18-1, East Yuquan Road, Yulu Village, Gongming Street, Guangming New District, Bao'an District, Shenzhen City, Guangdong Province, China, and the Registered Address at 3F, Building 1, No.18-1, East Yuquan Road, Yulu Village, Gongming Street, Guangming New District, Shenzhen City, Guangdong Province, China,
applicable to
has been assessed and registered by NQA against the provisions of Production of printed circuit board assemblies for electrocardiograph, endoscope
ISO 13485:2016.
This registration is subject to the company maintaining a quality management system to the above standard, which will be monitored by NQA. Certified Clients shall accept regular surveillance assessments, and the validity of certificates shall be maintained for the positive result of audit. The information of this certificate can be checked on CNCA's website (www.cnca.gov.cn) and SNQA's website (www.snqa.com.cn).
Shenzhen Jingbang Electronics Co., Ltd. (Unified Social Credit Code: 914403006925114418), with the Operation Address at 3F, Building 1, No.18-1, East Yuquan Road, Yulu Village, Gongming Street, Guangming New District, Bao'an District, Shenzhen City, Guangdong Province, China, and the Registered Address at 3F, Building 1, No.18-1, East Yuquan Road, Yulu Village, Gongming Street, Guangming New District, Shenzhen City, Guangdong Province, China,
applicable to
has been assessed and registered by NQA against the provisions of Production of printed circuit board assemblies for electrocardiograph, endoscope
ISO 13485:2016.
This registration is subject to the company maintaining a quality management system to the above standard, which will be monitored by NQA. Certified Clients shall accept regular surveillance assessments, and the validity of certificates shall be maintained for the positive result of audit. The information of this certificate can be checked on CNCA's website (www.cnca.gov.cn) and SNQA's website (www.snqa.com.cn).